Metabolic Associated Steatohepatitis
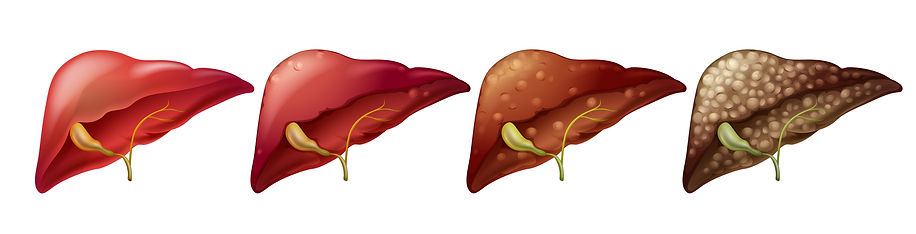
Nonalcoholic fatty liver disease (NAFLD) is a condition in which excess fat is stored in the liver. This buildup of fat is not caused by heavy alcohol use. Two types of NAFLD are nonalcoholic fatty liver (NAFL) and nonalcoholic steatohepatitis (NASH). People typically develop one type of NAFLD or the other, although sometimes people with one form are later diagnosed with the other form of NAFLD.
NAFLD, is a common and serious chronic liver disease, affects 25% of the population worldwide. NAFLD encompasses a spectrum of liver pathologies ranging from hepatic fat accumulation to NASH, characterized by liver cell damage, lobular inflammation, fibrosis and cirrhosis that can lead to liver failure or hepatocellular cancer. NAFLD is common in metabolic disease patients including those with obesity, type 2 diabetes (T2D), metabolic syndrome, dyslipidemia and atherosclerosis. Cardiovascular disease is the leading cause of death in NAFLD patients, particularly those with NASH. Despite the global burden of NAFLD and substantial efforts in drug development, no therapy has yet been approved.
NAFLD can affect people of any age, including children. Research suggests that close to 10% of U.S. children ages 2 to 19 have NAFLD. However, people are more likely to develop NAFLD as they age. While NAFLD occurs in people of all races and ethnicities, it is most common among Hispanic individuals, followed by non-Hispanic whites and Asian Americans, including those of East Asian and South Asian descent. NAFLD is less common among non-Hispanic Blacks. On average, Asian Americans with NAFLD have a lower BMI than non-Hispanic whites with NAFLD. Experts think that genes may help explain some of the racial and ethnic differences in NAFLD.
The NASH therapeutic area is active in most major pharma companies and is the focus of a significant number of small biotech companies. There are currently no approved products to treat NASH although diet and exercise have been shown to reverse moderate forms of the disease. GLP-1 receptor agonists are being used off label for this disease. Currently, there are 131 Phase II or Phase III, NASH trials underway or planned, and the resulting competition for qualified site resources as well as eligible patients is challenging for drug development companies. In addition, many of the drugs that have been in clinical development have failed for efficacy or safety reasons. Besides large Pharma companies, others such as Viking Therapeutics, Intercept Pharmaceuticals, and Madrigal Pharma have been very active in the field. These companies were able to make enough progress to go public and have large market capitalization. Investment in companies developing NASH therapies was strong, but recently has tapered off because of failures in the clinic.
Drugs that have failed in NASH clinical trials had done so due to lack of efficacy or safety issues. Several drugs in clinical trials have the same mechanism as compounds that have previously failed for NASH. The companies sponsoring these trials must believe that the mechanism was valid, but the specific compound had issues. Additional information on NAFLD/NASH can be found from the NIDDK (https://www.niddk.nih.gov/health-information/liver-disease/nafld-nash/definition-facts).
